Raising The Pulse
Investigating The Impact Of Faba Bean Rich Foods On Iron Status, Postprandial Lipaemia And Satiety In Adults
Purpose:
High levels of animal proteins (meat) are linked with a greater risk of developing heart disease and other long-term health conditions. Recently there has been a shift to plant-based diets including plant proteins such as pulses, defined as beans, peas, chickpeas and lentils. Pulses are a nutritious and sustainable form of plant protein which are rich in quality protein, fibre and iron. Despite this, the UK population does not consume the recommended amount of pulses (80g/day). In contrast, bread is commonly consumed but very little is known about how bread enriched with pulses influences the amount of iron that is digested and absorbed by the body as well as risk factors for developing heart disease and type 2 diabetes.The main purpose of this study is to determine how consuming bread enriched with pulses (in the form of pulse flour) compared with conventional white bread influences the amount of iron absorbed in healthy males and females. Secondary aims are determining the effects of these different breads on blood fats and sugar (glucose) and on feelings of fullness (also known as satiety).
Who can take part in this study?
We aim to recruit 16 males and premenopausal females between the ages of 18 and 50 years. Suitable volunteers should
- have a body mass index of 19-30 kg/m2
- be non-smokers
- have low iron stores in the body but not be anaemic.
Exclusion criteria
Males and females who will not be able to take part in the study include those diagnosed with
- anaemia
- haemochromatosis (very high levels of iron in the blood)
- diabetes
- heart disease (previous stroke or heart attack) or a pacemaker
- kidney, bowel or liver or gastrointestinal (gut) diseases, cancer or hormone abnormalities.
We must also exclude females who are peri- or postmenopausal, pregnant or planning a pregnancy within the next six months, lactating or breastfeeding.
We must also exclude those
- with food allergies or an intolerance to faba beans (known as favism),
- taking certain types of medication (e.g. for anaemia, high blood pressure, high blood fats, inflammatory conditions and depression), or dietary supplements (e.g. vitamin or mineral supplements),
- who are actively trying to lose or who have lost more than 3 kg of weight in the last 6 months.
Volunteers who have participated in another intervention study or donated blood in the last 3 months will be also excluded.
What will taking part in the study involve?
Screening visit
- Initial screening visit at the Hugh Sinclair Unit of Human Nutrition in the Department of Food & Nutritional Sciences (University of Reading). The screening will be scheduled in the morning and you will be asked to arrive fasted. The screening visit should take no more than 40 minutes and you will be provided with a light breakfast before you leave.
- If you are found suitable for the study and are willing to participate, we will confirm with you your participation in the study. If you have any abnormal results, these will be reported to you and your GP.
Study visits
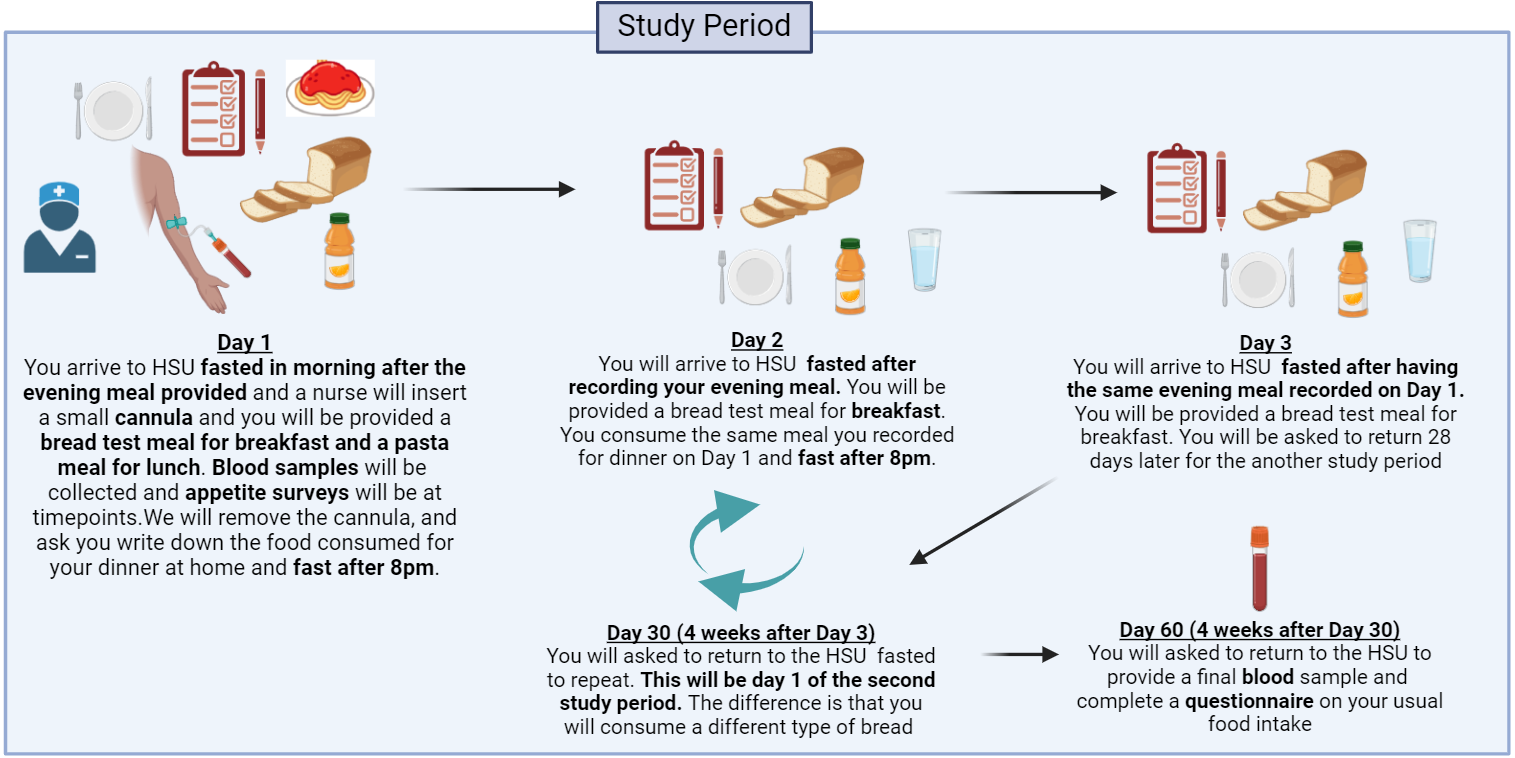
- There will be two study periods consisting of 3 days each, over 3 consecutive days, with a gap of 4 weeks between the two study periods.
- During the study periods, you will be given test meals for 3 consecutive days (breakfast and lunch on Day 1 and only breakfast on Days 2 and 3). Breakfast will be toasted bread with margarine and chocolate spread, orange juice and water. The type of bread consumed during each study period will be randomly assigned by the researchers and you will not know the order. Volunteers will be required to attend all the study visits.
- On Day 1 of both study periods a small flexible cannula will be inserted into a vein in your arm by a research nurse and this will remain in place with minimal discomfort to allow us to take blood samples during the study day.
- For the final study visit, 4 weeks after Day 3 of the second study period, you will be asked to arrive fasted overnight. On arrival, your weight, body composition (e.g. percentage body fat) and blood pressure will be measured, before a fasting blood sample is collected (20 ml, volume equivalent to approximately 1 ½ tablespoons). You will be provided with a snack before you go home and that will be the study fully completed.
Study contact
Please contact the study researchers on rtp3@reading.ac.uk
